 |
OSOM® H. pylori Test
SEKISUI DIAGNOSTICS
Detects IgG antibodies to H. pylori in serum, plasma or whole blood. CLIA-waived for whole blood. 95.9% sensitivity. Result in 10 minutes or less. 18 month room temperature storage.
|
|||||||||||||||
|
For the qualitative detection of Helicobacter pylori antibodies in serum, plasma or whole blood as an aid in the diagnosis of H. pylori infection. Features and Benefits
SEKISUI DIAGNOSTICS
4 Hartwell Place | | Lexington | MA |
For over 35 years Sekisui Diagnostics has been committed to providing innovative medical diagnostics to physicians and laboratories. We develop, manufacture, and supply billions of tests each year to the global healthcare market. Our product lines include clinical chemistry and coagulation systems and reagents, point-of-care molecular, rapid tests and immunoassay system as well as enzymes and specialty biochemicals.

|
||||||||||||||||
 |
Alere Cholestech LDX® Analyzer
ABBOTT RAPID DIAGNOSTICS
The CLIA-waived Alere Cholestech LDX® Analyzer is engineered for confidence, providing accurate, actionable, and readily accessible results that have set the standard in point-of-care lipid profile, cholesterol, and glucose testing.
|
|||||||||||||||
|
Accurate, actionable results from the leader in point-of-care lipid testing. The CLIA-waived Alere Cholestech LDX® Analyzer is engineered for confidence, providing accurate, actionable, and readily accessible results that have set the standard in point-of-care lipid profile, cholesterol, and glucose testing. Test fasting lipid profile: total cholesterol (TC), HDL & LDL cholesterol, and triglycerides (TRG) for risk assessment and lipid management. The Alere Cholestech LDX® System provides lab accurate results in just five minutes from a small fingerstick sample. Benefits of the Alere Cholestech LDX® Analyzer:
Simple 3-Step Testing: Testing full lipid profile and glucose is as easy as 1, 2, 3:
Relevant Clinical Areas:


Click Images to View Larger
ABBOTT RAPID DIAGNOSTICS
xxxx my street | | concord | NH |
Abbott is a world leader in rapid diagnostics at the point of care, with a focus on cardio metabolic disease, infectious disease and toxicology.

|
||||||||||||||||
 |
Afinion™ 2 Analyzer
ABBOTT RAPID DIAGNOSTICS
The Afinion™ 2 analyzer is a compact, rapid, multi-assay analyzer that provides valuable near patient testing at the point-of-care.
|
|||||||||||||||
|
Afinion™ 2 Simply More Efficient. Improve the way you monitor and manage your patients The Afinion™ 2 analyzer is a compact, rapid, multi-assay analyzer that provides valuable near patient testing at the point-of-care. With the Afinion™ System, there's no need to send test results to the lab or spend time tracking them down. That way you can keep your focus where it belongs – on the patient. Benefits of the Afinion™ 2 Analyzer: Multiple analytes on one instrument
3-Step Procedure
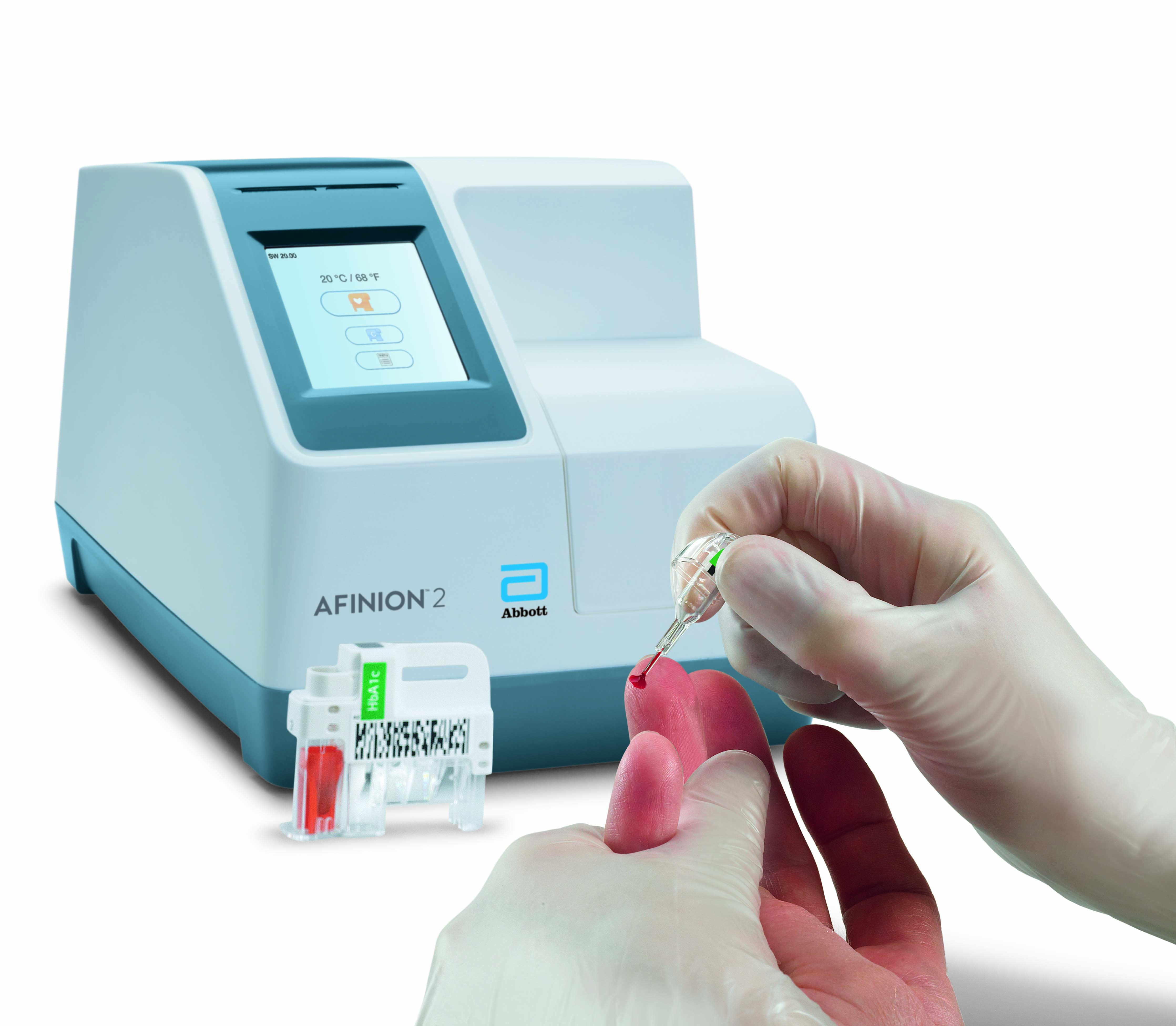
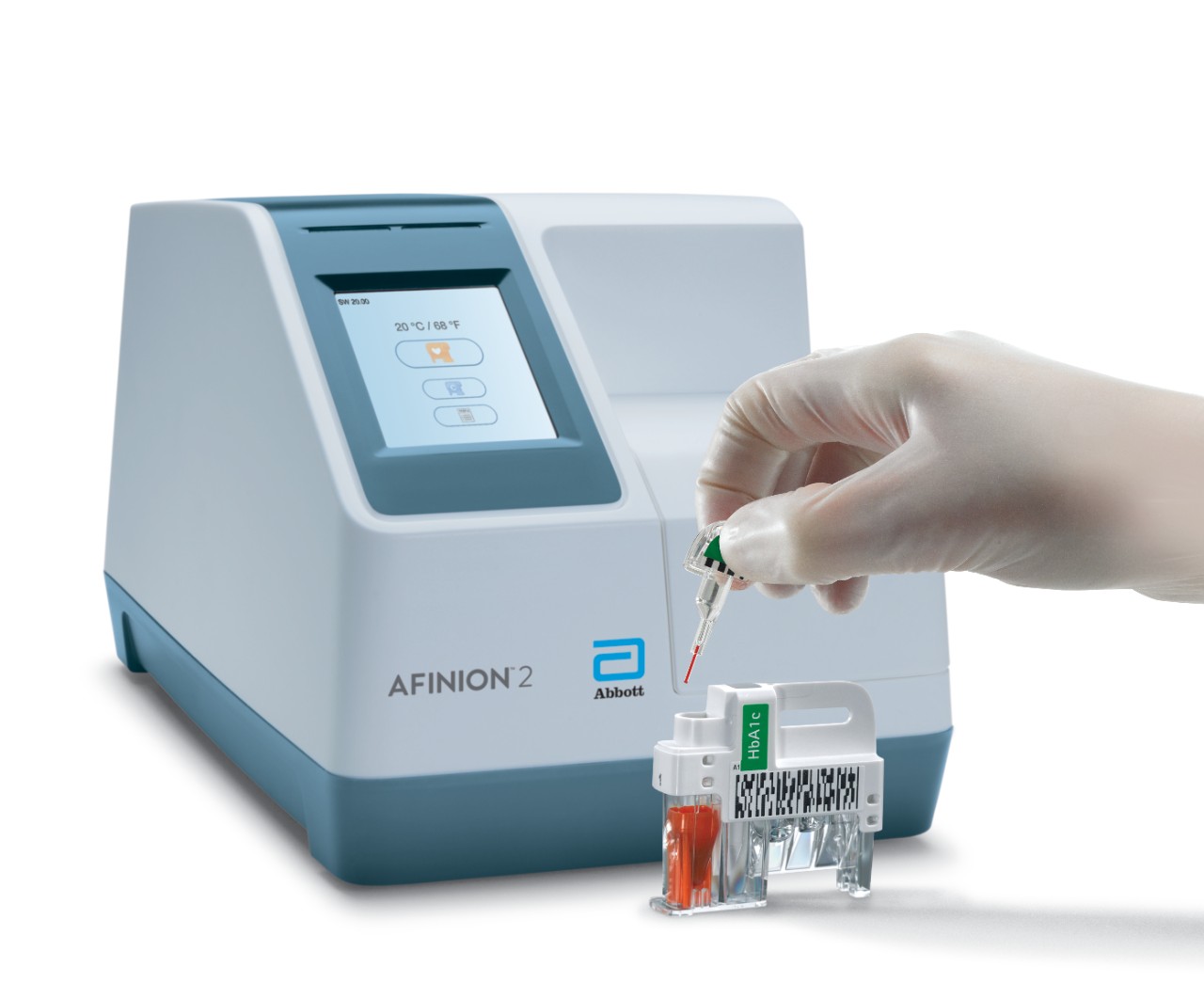
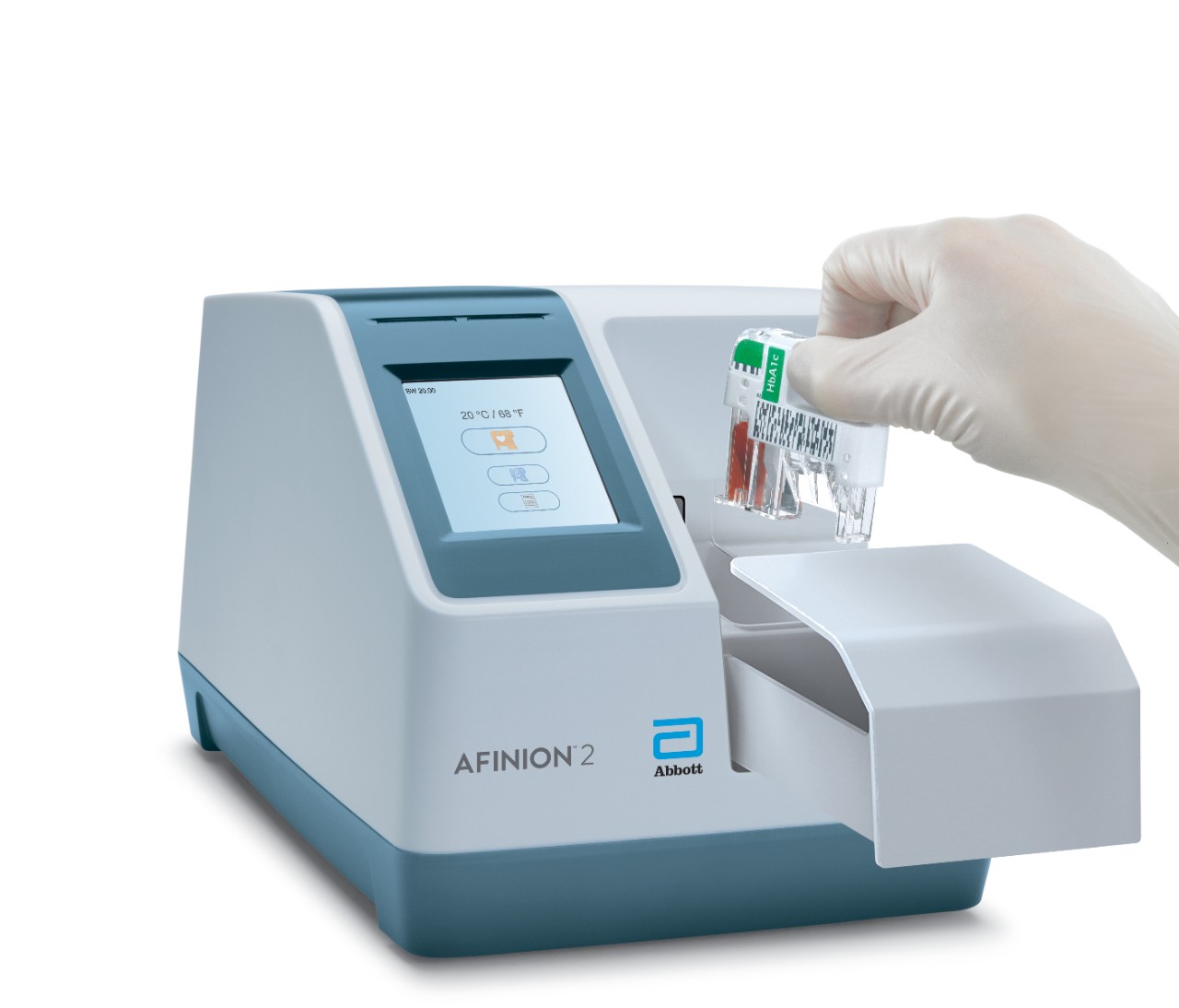
Click Images to View Larger
ABBOTT RAPID DIAGNOSTICS
xxxx my street | | concord | NH |
Abbott is a world leader in rapid diagnostics at the point of care, with a focus on cardio metabolic disease, infectious disease and toxicology.

|
||||||||||||||||
 |
CoaguChek XS Plus
ROCHE DIAGNOSTICS
Smart PT/INR monitoring at the physician's practice. Healthcare professionals can measure a patient's PT/INR value usually in less than 1 minute with a drop of capillary blood (8 µl).
|
|||||||||||||||
|
CoaguChek® XS Plus Simplified testing with enhanced connectivity for professional use This small, battery-powered, handheld meter is portable and efficient, ideal for mid to high-volume clinical settings. Plus, you can test and treat in one appointment, using the fingerstick test that patients prefer. Accurate
Easy to use
Smart
*97% correlation with lab results using Dade Innovin reagent on a Sysmex 560 Analyzer. See package insert for more information. CoaguChek XS PT Test [package insert 05967694001(03)]. Indianapolis, Ind.: Roche Diagnostics; 2013
ROCHE DIAGNOSTICS
9115 Hague Road | Bldg. H | Indianapolis | IN |
Personalised Helathcare (PHC) is based on the observation that patients with the same diagnosis react to the same treatment in different ways

|
||||||||||||||||
 |
CoaguChek® XS
ROCHE DIAGNOSTICS
Only handheld, CLIA-waived PT/INR monitoring system that neutralizes therapeutic levels of heparin and low molecular weight heparin within a specified range
|
|||||||||||||||
|
CoaguChek® XS Meter Accurate
Easy to use
*97% correlation with lab results using Dade Innovin reagent on a Sysmex 560 Analyzer. See package insert for more information. CoaguChek XS PT Test [package insert 05967694001(03)]. Indianopolis, IND.: Roche Diagnostics; 2013
ROCHE DIAGNOSTICS
9115 Hague Road | Bldg. H | Indianapolis | IN |
Personalised Helathcare (PHC) is based on the observation that patients with the same diagnosis react to the same treatment in different ways

|
||||||||||||||||
 |
cobas® Influenza A/B assay
ROCHE DIAGNOSTICS
The cobas Influenza A/B assay is the first CLIA-waived, real-time PCR test to detect Influenza A and B in ~20 minutes
|
|||||||||||||||
|
cobas® Influenza A/B assay The cobas® Influenza A/B assay is the first CLIA-waived, real-time PCR test to detect Influenza A and B in ~20 minutes. Available for use in non-traditional testing sites, including ERs, physician offices, pharmacy clinics and other urgent care settings. Benefits:
ROCHE DIAGNOSTICS
9115 Hague Road | Bldg. H | Indianapolis | IN |
Personalised Helathcare (PHC) is based on the observation that patients with the same diagnosis react to the same treatment in different ways

|
||||||||||||||||
 |
cobas® Strep A assay
ROCHE DIAGNOSTICS
The cobas Strep A assay is a CLIA-waived, real-time PCR test for the detection of Streptococcus group A in throat swab specimens from patients with signs and symptoms of pharyngitis.
|
|||||||||||||||
|
cobas® Strep A assay The cobas® Strep A assay is a CLIA-waived, real-time PCR test for the detection of Streptococcus group A in throat swab specimens from patients with signs and symptoms of pharyngitis. With excellent sensitivity and specificity, this assay provides the reassurance needed when deciding whether or not to treat with antibiotics. Benefits:
ROCHE DIAGNOSTICS
9115 Hague Road | Bldg. H | Indianapolis | IN |
Personalised Helathcare (PHC) is based on the observation that patients with the same diagnosis react to the same treatment in different ways

|
||||||||||||||||
 |
cobas® Influenza A/B & RSV assay
ROCHE DIAGNOSTICS
The cobas Influenza A/B & RSV assay is the first CLIA-waived, real-time PCR test that differentiates Influenza A, Influenza B and RSV in 20 minutes
|
|||||||||||||||
|
cobas® Influenza A/B & RSV assay The cobas Influenza A/B & RSV assay is the first CLIA-waived, real-time PCR test that differentiates Influenza A, Influenza B and RSV in 20 minutes. It's available for use in hospitals, physician offices and urgent care settings. Benefits:
ROCHE DIAGNOSTICS
9115 Hague Road | Bldg. H | Indianapolis | IN |
Personalised Helathcare (PHC) is based on the observation that patients with the same diagnosis react to the same treatment in different ways

|
||||||||||||||||
 |
BTA stat Test
POLYMEDCO, INC.
The BTA stat Test is an in vitro immunoassay intended for the qualitative detection of bladder tumor antigen in urine of persons diagnosed with bladder cancer. This test is indicated for use as an aid in the management of bladder cancer patients in conjunction with cytology.
|
|||||||||||||||
|
The BTA stat® test is a point of care technology for the early detection of recurrent bladder cancer. This method uses monoclonal antibodies to detect the presence of bladder tumor associated antigen in urine. It is a single-step, rapid immunochromatographic assay for bladder tumor-associated antigen in voided urine. The specificity of the BTA stat® test was 93 - 95% in patients with non-genitourinary diseases and cancers and healthy individuals tested as part of a multi-center study. The test has a sensitivity that is considerably higher than voided urine cytology, enabling detection of recurrent early stage and grade cancers that cytology often misses. Requiring three drops of urine, the result is delivered in only five minutes. The appearance of a line in the patient window indicates a positive result. The BTA stat® test requires one voided urine sample with no sample preparation. The BTA stat® test is CLIA waived and also available for prescription home use.
POLYMEDCO, INC.
510 Furnace Dock Road | | Cortlandt Manor | NY |
Polymedco Inc. is a leading manufacturer, marketer, and distributer in the clinical laboratory marketplace. Polymedco supplies clinical diagnostic test kits and devices that are specialized in chemistry, hematology and cancer.

|
||||||||||||||||
 |
A1CNow+
BAYER HEALTHCARE
A1CNow+ is a portable, easy-to-use system that provides on-the-spot HbA1c result in 5 minutes. This enables the healthcare provider to make immediate treatment decisions and discuss them with patients face-to-face during their visits. The A1CNow+ is portable which enables testing in multiple exam rooms and requires no capital investment. No maintenance, CLIA waived, NGSP certified and reimbursable.
|
|||||||||||||||
|
Purchasing A1CNow+
BAYER HEALTHCARE
510 Oakmead Parkway | | Sunnyvale | CA |
Bayer's A1CNow provides a fast and easy way of obtaining accurate A1C results.

|
||||||||||||||||